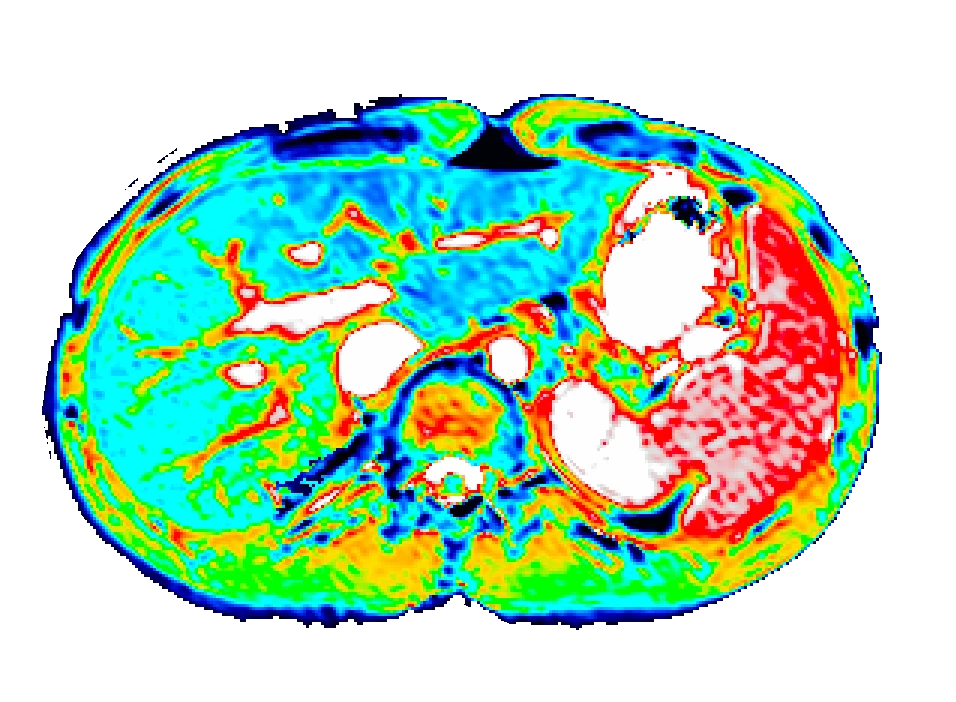
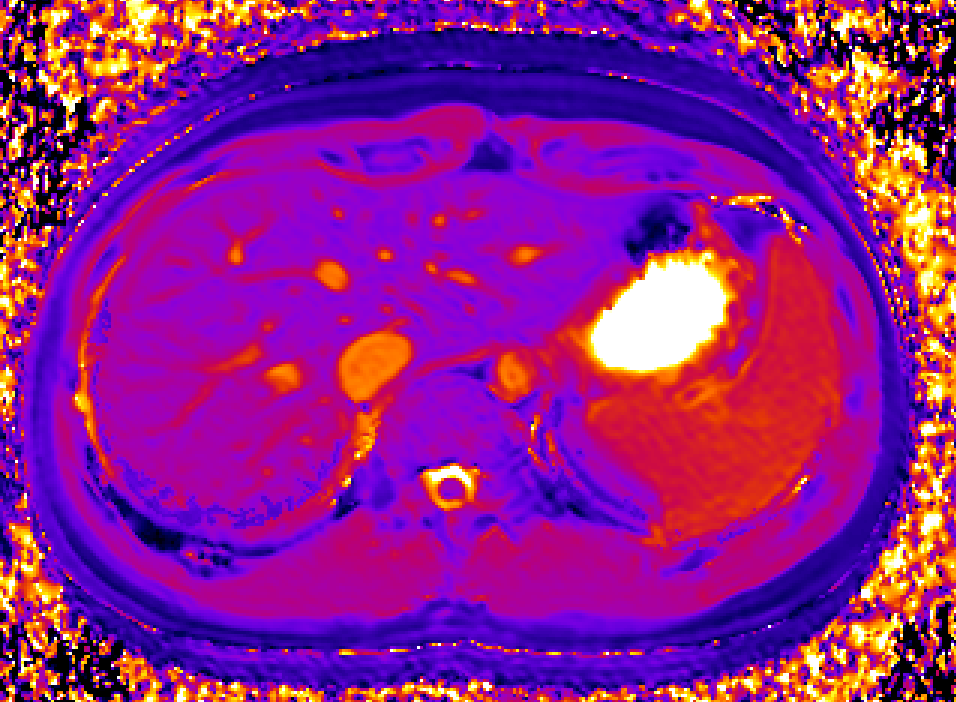
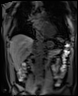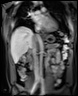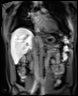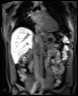
Bioxydyn is the lead commercial partner in the IMI TRISTAN project, which is investigating the liver and lung toxicity of many drugs in use today with the goal of validating the use of imaging biomarkers to assess and predict the toxicity of potential medicines on the liver and lungs.
The TRISTAN consortium aimed to develop and validate gadoxetate-based DCE-MRI biomarkers for DILI and DDI. Drug developers and regulatory authorities are more likely to rely on such imaging biomarkers if they trust the acquisition, analysis and interpretation. For this reason we have developed a precise specification which has been accepted into FDA’s biomarker qualification programme (BQP): "the change, caused by investigational (perpetrator) drug, in gadoxetate (victim drug surrogate) hepatocyte uptake and efflux rate constants [∆k(he) and ∆k(bh), respectively] indicating potential for drug-drug interaction when above threshold" and the context is use is: "a safety (acute lack-of-harm) biomarker, employed in the development of investigational drugs (IDs) which are thought to carry an enhanced DDI risk because prior animal or in vitro human cell studies have indicated that the ID is a hepatic transporter inhibitor or inducer. ∆k(he) and ∆k(bh) would be used in early phase clinical drug development. An ID whose effect on either ∆k(he) or ∆k(bh) is not below-threshold would be prioritized for an early program of clinical DDI investigations".
Our platform of evidence includes a range of in vitro, rat, and human studies of the reproducibility of the assay and its response to multiple known transporter substrates.